
Chemistry, 14.04.2020 22:20 casting479
A sample of hydrogen gas occupies a volume of 6.38 L at 48.0°C and 0.490 atm. If it is desired to increase the volume of the gas sample to 8.10 L, while increasing its pressure to 0.655 atm, the temperature of the gas sample at the new volume and pressure must be °C.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 18:00
Mercury turns to vapor at 629.88 k how much heat is lost 175 g of mercury vapor at 650 current condenses to a liquid at 297 ca mercury turns to weber at 629.88 kelvin how much he is lost 175 g of mercury vapor and 650 coming condensers to liquidate 297 kevin
Answers: 2

Chemistry, 22.06.2019 23:30
Imagine a small synthetic vesicle made from pure phospholipids enclosing an interior lumen containing 1 mm glucose and 1 mm sodium chloride. if the vesicle is placed in pure water, which of the following happens faster? a. na+ diffuses out. b. cl– diffuses out. c. h2o diffuses in. d. glucose diffuses out. e. sodium chloride diffuses out.
Answers: 3

Chemistry, 23.06.2019 00:00
How many moles of water are created if 3 moles of hydrogen react completely with excess oxygen?
Answers: 3

Chemistry, 23.06.2019 06:00
When hydrogen peroxide (h2o2) is added to potassium iodide (ki) solution, the hydrogen peroxide decomposes into water (h2o) and oxygen (o2). the chemical equation for the decomposition reaction is: 2h2o2—> 2h2o + o2. what is the role of the potassium iodide in this reaction? a. reactant. b. product. c. precipitate. d. catalyst.
Answers: 1
You know the right answer?
A sample of hydrogen gas occupies a volume of 6.38 L at 48.0°C and 0.490 atm. If it is desired to in...
Questions

Mathematics, 12.05.2020 15:57

Mathematics, 12.05.2020 15:57


Mathematics, 12.05.2020 15:57

English, 12.05.2020 15:57



Computers and Technology, 12.05.2020 15:57






Physics, 12.05.2020 15:57





History, 12.05.2020 15:57

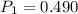 atm
atm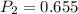 atm
atm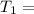 48° C = 321 K
48° C = 321 K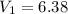 L
L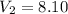 L
L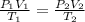
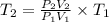
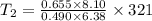
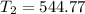 K
K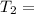 271.77°C
271.77°C

