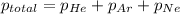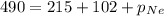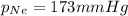Chemistry, 14.04.2020 22:18 shaheedbrown06
A tank contains a mixture of helium, neon, and argon gases. If the total pressure in the tank is 490. mmHg and the partial pressures of helium and argon are 215 mmHg and 102 mmHg, respectively, what is the partial pressure of neon

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:00
The continuous release of nuclear energy caused when one fission reaction triggered more nuclear reactions is a
Answers: 3

Chemistry, 22.06.2019 14:30
Aroom with dimensions 7.00m×8.00m×2.50m is to be filled with pure oxygen at 22.0∘c and 1.00 atm. the molar mass of oxygen is 32.0 g/mol. how many moles noxygen of oxygen are required to fill the room? what is the mass moxygen of this oxygen?
Answers: 1

Chemistry, 22.06.2019 19:00
Convert the temperature of dry ice, –77 ∞c, into degrees fahrenheit and kelvin.
Answers: 2

Chemistry, 22.06.2019 22:30
The vapor pressure of ethanol is 1.00 × 102 mmhg at 34.90°c. what is its vapor pressure at 61.61°c? (δhvap for ethanol is 39.3 kj/mol.)
Answers: 2
You know the right answer?
A tank contains a mixture of helium, neon, and argon gases. If the total pressure in the tank is 490...
Questions


Mathematics, 07.10.2019 02:30

Health, 07.10.2019 02:30

Social Studies, 07.10.2019 02:30




History, 07.10.2019 02:30

Mathematics, 07.10.2019 02:30



Advanced Placement (AP), 07.10.2019 02:30

Social Studies, 07.10.2019 02:30



Mathematics, 07.10.2019 02:50

Biology, 07.10.2019 02:50


History, 07.10.2019 02:50

Mathematics, 07.10.2019 02:50

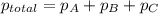
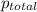 =total pressure of gases = 490 mm Hg
=total pressure of gases = 490 mm Hg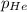 = partial pressure of Helium = 215 mmHg
= partial pressure of Helium = 215 mmHg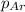 = partial pressure of argon = 102 mm Hg
= partial pressure of argon = 102 mm Hg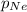 = partial pressure of neon= ?
= partial pressure of neon= ?