
Chemistry, 14.04.2020 21:12 larapoghosyan91
Valeric acid, HC5H9O2 (Ka = 1.5 ✕ 10−5), is used in the manufacture of magnesium valerate, a nerve-calming agent. What is the hydronium ion concentration (in M) in a 0.737 M solution of HC5H9O2? (Assume Kw = 1.01 ✕ 10−14.)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 13:00
The molality of calcium chloride (cacl2) in an aqueous solution is 2.46 m. what is mole fraction of the solute?
Answers: 3

Chemistry, 23.06.2019 00:30
What are the advantages of using the metric system? designed as a decimal system making conversions simpler more accurate system of measurement has prefixes that correspond to an amount to use with all base units used by the entire scientific community
Answers: 2

Chemistry, 23.06.2019 01:30
Use the periodic table to determine how many grams of oxygen would be required to react completely with 859.0 g c2h2
Answers: 3

Chemistry, 23.06.2019 01:30
The biomedical technique in which a part of the brain is destroyed with electric current is known as a. electroconvulsive therapy b. prefrontal lobotomy c. bilateral cingulotomy d. tardive dyskinesia
Answers: 2
You know the right answer?
Valeric acid, HC5H9O2 (Ka = 1.5 ✕ 10−5), is used in the manufacture of magnesium valerate, a nerve-c...
Questions





Mathematics, 24.07.2019 04:00

History, 24.07.2019 04:00


Arts, 24.07.2019 04:00

Mathematics, 24.07.2019 04:00

Mathematics, 24.07.2019 04:00


Mathematics, 24.07.2019 04:00


Physics, 24.07.2019 04:00

Physics, 24.07.2019 04:00

Mathematics, 24.07.2019 04:00


Chemistry, 24.07.2019 04:00


Mathematics, 24.07.2019 04:00

![[H^+]=0.00332M](/tpl/images/0599/2796/eac47.png)
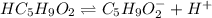
![Ka=\frac{[H^+][C_5H_9O_2^-]}{[HC_5H_9O_2]}](/tpl/images/0599/2796/6b49e.png)
 due to dissociation, it becomes:
due to dissociation, it becomes: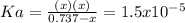
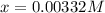
![[H^+]=x=0.00332M](/tpl/images/0599/2796/13d16.png)


