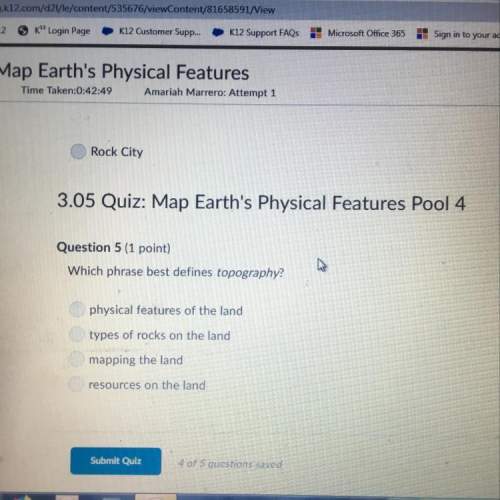
Chemistry, 14.04.2020 21:05 WhiteMex69
A copper block (20°C) is placed in contact with a lead block (30°C), which is already in contact with an iron block (70°C). What will happen in this situation? Heat will flow from the copper to the lead and from the iron to the lead until the temperatures are equal. Heat will flow from the lead to the copper and to the iron until the temperatures are equal. Heat will flow from the iron to the lead and from the lead to the copper until the temperatures are equal. Heat will flow from the copper to the lead to the iron until the temperatures are equal.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:00
The human eye contains a molecule called 11-cis-retinal that changes shape when struck with light of sufficient energy. the change in shape triggers a series of events that results in an electrical signal being sent to the brain that results in vision. the minimum energy required to change the conformation of 11-cis-retinal within the eye is about 164 kj/mol.
Answers: 2

Chemistry, 22.06.2019 12:30
Consider the four elements above. which one of these elements will combine with oxygen in a 1: 1 ratio?
Answers: 3

Chemistry, 23.06.2019 03:00
What volume does 1.70 ×10–3 mol of chlorine gas occupy if its temperature is 20.2 °c and its pressure is 795 mm hg?
Answers: 3

Chemistry, 23.06.2019 04:31
2ki + pb(no3)2 → 2kno3 + pbi2 determine how many moles of kno3 are created if 0.03 moles of ki are completely consumed.
Answers: 1
You know the right answer?
A copper block (20°C) is placed in contact with a lead block (30°C), which is already in contact wit...
Questions


Business, 06.10.2019 07:11

Mathematics, 06.10.2019 07:11

Mathematics, 06.10.2019 07:11


Mathematics, 06.10.2019 07:11

Spanish, 06.10.2019 07:11

English, 06.10.2019 07:11


English, 06.10.2019 07:11




Mathematics, 06.10.2019 07:11

History, 06.10.2019 07:11

History, 06.10.2019 07:11


Health, 06.10.2019 07:11

Mathematics, 06.10.2019 07:11