What is the molarity of a solution of HCl if 5.00 mL of the HCl solution is
titrated with 28.6...

Chemistry, 14.04.2020 20:43 pippyysanchezz
What is the molarity of a solution of HCl if 5.00 mL of the HCl solution is
titrated with 28.6 mL of a 0.145 M NaOH solution?
Round your answer to 3
decimal places.
HCl + NaOH → NaCl + H 2 O

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 13:30
What does the xylem do? stores the glucose captures the sunlight absorbs oxygen into the leaf carries water from the roots to the leaves
Answers: 1

Chemistry, 22.06.2019 19:30
Phosphorous can form an ion called phosphide, which has the formula p3−. this ion can form an ion called phosphide, which has the formula p3−. this ion properties very similar to those of pforms when a phosphorus atom loses three protonsis called a cationcontains 18 electrons
Answers: 2

Chemistry, 22.06.2019 20:30
Select all the correct answers.which compounds have the empirical formula ch20? (multiple answers)a.c2h4o2b.c3h603c.ch2o2d.c5h1005e.c6h1206
Answers: 2

Chemistry, 23.06.2019 03:00
In which of the following phases of matter do molecules have the highest amount of energy? a. liquid b. gel c. solid d. gas
Answers: 2
You know the right answer?
Questions

History, 19.04.2021 20:40

SAT, 19.04.2021 20:40


Mathematics, 19.04.2021 20:40


Mathematics, 19.04.2021 20:40



Chemistry, 19.04.2021 20:40

Mathematics, 19.04.2021 20:40


Mathematics, 19.04.2021 20:40

Computers and Technology, 19.04.2021 20:40






Social Studies, 19.04.2021 20:40

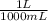 ) x (
) x (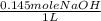 ) x (
) x (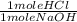 ) = 0.004147 moles HCl
) = 0.004147 moles HCl

