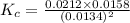Chemistry, 14.04.2020 20:01 dontworry48
Consider the following reaction: 2CH2Cl2(g) CH4(g) CCl4(g) If 0.203 moles of CH2Cl2(g), 0.323 moles of CH4, and 0.240 moles of CCl4 are at equilibrium in a 15.2 L container at 477 K, the value of the equilibrium constant, Kc, is

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 17:30
Which scenario is most similar to the type of collision that gas particles have according to kinetic molecular theory
Answers: 1

Chemistry, 22.06.2019 23:30
With the largest atoms and the smallest number of valence electrons and with the smallest atoms and the greatest number of valence electrons are the most reactive. a. nonmetals; metals b. nonmetals; transition elements c. transition elements; metals d. metals; nonmetals
Answers: 3

Chemistry, 23.06.2019 00:00
Which is true about metals used for jewelry, such as platinum and gold? a. they have low flammability. b. they have low reactivity. c. they have high flammability. d. they have high reactivity.
Answers: 1

Chemistry, 23.06.2019 05:00
Match each term to its description. match term definition excess reactant a) reactant that can produce a lesser amount of the product limiting reactant b) amount of product predicted to be produced by the given reactants theoretical yield c) reactant that can produce more of the product
Answers: 3
You know the right answer?
Consider the following reaction: 2CH2Cl2(g) CH4(g) CCl4(g) If 0.203 moles of CH2Cl2(g), 0.323 moles...
Questions


Computers and Technology, 16.05.2021 05:50

Computers and Technology, 16.05.2021 05:50



History, 16.05.2021 05:50




Mathematics, 16.05.2021 05:50


Mathematics, 16.05.2021 05:50


Mathematics, 16.05.2021 05:50


Physics, 16.05.2021 05:50


English, 16.05.2021 05:50

Mathematics, 16.05.2021 05:50

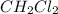 at equilibrium= 0.203 mole
at equilibrium= 0.203 mole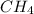 at equilibrium = 0.323 mole
at equilibrium = 0.323 mole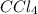 at equilibrium = 0.240mole
at equilibrium = 0.240mole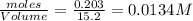
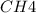 =
= 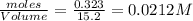
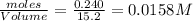
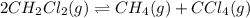
![K_c=\frac{[CH_4]\times [CCl_4]}{[CH_2Cl_2]^2}](/tpl/images/0599/0518/28fa5.png)