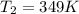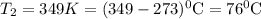Mount Everest rises to a height of 8,850 m above sea level. At a base camp on the mountain the atmospheric pressure is measured to be 314.0 mm Hg. At what temperature (in °C) will water boil at base camp ? The vapor pressure of water at 373 K is 760.0 mm Hg. (ΔH°vap for H2O = 40.7 kJ/mol and R = 8.314 J/mol K).
a. 344°C.
b. 70.8°C .
c. 2.91E-3°C .
d. 79.8°C.
e. 57.8°C.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Sex cells from female reproductive organ? 1) mitosis 2) fertilization 3) zygote 4) eggs 5) meiosis 6) sperm
Answers: 2

Chemistry, 22.06.2019 10:00
Part 1: include important facts found through your research. part 2: include your visual display. include your summary of “the chemistry of water” from the national science foundation website. include your experiment. part 3: include responses to the reflection questions.
Answers: 1

Chemistry, 22.06.2019 13:30
How many protons, electrons, and neutrons are in each of the following isotopes? a. zirconium-90 b. palladium-108 c. bromine-81 d. antimony-123
Answers: 1

You know the right answer?
Mount Everest rises to a height of 8,850 m above sea level. At a base camp on the mountain the atmos...
Questions

History, 16.10.2019 04:00





Mathematics, 16.10.2019 04:00


Mathematics, 16.10.2019 04:00

Mathematics, 16.10.2019 04:00


Mathematics, 16.10.2019 04:00


English, 16.10.2019 04:00


Mathematics, 16.10.2019 04:00

English, 16.10.2019 04:00



Mathematics, 16.10.2019 04:00

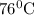 .
.![ln(\frac{P_{2}}{P_{1}})=\frac{-\Delta H_{vap}^{0}}{R}[\frac{1}{T_{2}}-\frac{1}{T_{1}}]](/tpl/images/0599/0769/06906.png)
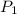 and
and 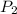 are vapor pressures of liquid at
are vapor pressures of liquid at 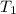 (in kelvin) and
(in kelvin) and 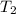 (in kelvin) temperatures respectively.
(in kelvin) temperatures respectively.![ln(\frac{314.0}{760.0})=\frac{-40.7\times 10^{3}\frac{J}{mol}}{8.314\frac{J}{mol.K}}\times [\frac{1}{T_{2}}-\frac{1}{373K}]](/tpl/images/0599/0769/811c1.png)