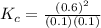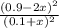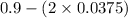Chemistry, 14.04.2020 19:52 KariSupreme
At equilibrium, the concentrations in this system were found to be [ N 2 ] = [ O 2 ] = 0.100 M [N2]=[O2]=0.100 M and [ NO ] = 0.600 M . [NO]=0.600 M. N 2 ( g ) + O 2 ( g ) − ⇀ ↽ − 2 NO ( g ) N2(g)+O2(g)↽−−⇀2NO(g) If more NO NO is added, bringing its concentration to 0.900 M, 0.900 M, what will the final concentration of NO NO be after equilibrium is re‑established?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:30
There are main groups in the modern periodic table of elements
Answers: 1

Chemistry, 22.06.2019 05:30
What is the mass of each element in a 324.8 sample of co2
Answers: 1

Chemistry, 22.06.2019 12:20
The yearly amounts of carbon emissions from cars in belgium are normally distributed with a mean of 13.9 gigagrams per year and a standard deviation of 5.8 gigagrams per year. find the probability that the amount of carbon emissions from cars in belgium for a randomly selected year are between 11.5 gigagrams and 14.0 gigagrams per year. a. 0.340 b. 0.660 c. 0.167 d. 0.397
Answers: 2

Chemistry, 23.06.2019 03:00
Select the correct answer. wax is a nonpolar substance. in which type of substance is it most soluble?
Answers: 2
You know the right answer?
At equilibrium, the concentrations in this system were found to be [ N 2 ] = [ O 2 ] = 0.100 M [N2]=...
Questions


Mathematics, 28.04.2022 01:00

World Languages, 28.04.2022 01:00


Business, 28.04.2022 01:00

Spanish, 28.04.2022 01:00






Mathematics, 28.04.2022 01:20

Mathematics, 28.04.2022 01:20


Computers and Technology, 28.04.2022 01:30


SAT, 28.04.2022 01:50


Business, 28.04.2022 01:50

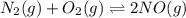
![K_{c} = \frac{[NO]^{2}}{[N_{2}][O_{2}]}](/tpl/images/0599/0103/a7a07.png)
![[N_{2}] = [O_{2}]](/tpl/images/0599/0103/c9b74.png) = 0.1 M and [NO] = 0.6 M
= 0.1 M and [NO] = 0.6 M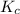 is as follows.
is as follows.