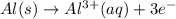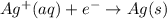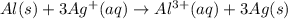Type the half-cell reaction that takes place at the anode for the aluminum-silver voltaic cell. Indicate the physical states of atoms and ions using the abbreviation (s), (l), or (g) for solid, liquid, or gas, respectively. Use (aq) for an aqueous solution. Do not include phases for electrons.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:00
To save time, you can approximate the initial mass of the solid to the nearest ±1 g. for example, if you are asked to add 14.3 g of copper, add between 13 g and 15 g. which of the following sets include two samples with an equal density? which all that apply below 15.4 g gold and 18.7 g silver 15.2 g copper and 50.0 g copper 20.2 g silver and 20.2 g copper 11.2 g gold and 14.9 g gold
Answers: 1

Chemistry, 21.06.2019 23:00
A100-watt light bulb radiates energy at a rate of 100 j/s. (the watt, a unit of power or energy over time, is defined as 1 j/s.) if all of the light emitted has a wavelength of 525 nm , how many photons are emitted per second?
Answers: 1

Chemistry, 22.06.2019 09:00
George is a dalmatian puppy. describe what happens to light that allows you to see george’s black and white coat.
Answers: 1

Chemistry, 22.06.2019 14:30
Connect the whole numbers on the periodic table to indicate what they represent?
Answers: 3
You know the right answer?
Type the half-cell reaction that takes place at the anode for the aluminum-silver voltaic cell. Indi...
Questions



Health, 15.04.2021 18:30

Mathematics, 15.04.2021 18:30


Mathematics, 15.04.2021 18:30

History, 15.04.2021 18:30

English, 15.04.2021 18:30


Mathematics, 15.04.2021 18:30


Mathematics, 15.04.2021 18:30

Mathematics, 15.04.2021 18:30



History, 15.04.2021 18:30



Mathematics, 15.04.2021 18:30

Biology, 15.04.2021 18:30