
Chemistry, 14.04.2020 19:26 oldless504
A sample of gallium bromide, GaBr3, weighing 0.165 g was dissolved in water and treated with silver nitrate, AgNO3, resulting in the precipitation of 0.299 g AgBr. Use these data to compute the %Ga (by mass) GaBr3.

Answers: 3


Another question on Chemistry

Chemistry, 20.06.2019 18:04
What is the molarity of a solution that is prepared by adding 57.1 g of toluene (c 7 h 8 ) (density = 0.867 g/ml) to a 250 ml volumetric flask, and then filling to the mark with benzene (c 6 h 6 ) (density = 0.876 g/ml)?
Answers: 1


Chemistry, 22.06.2019 13:00
The number of neutrons is equal to the atomic number minus the atomic mass. a. true b. false
Answers: 2

Chemistry, 22.06.2019 16:30
An atom with 7 protons, 6 neutrons, and 7 electrons has an atomic mass of amu. (enter a whole number.) numerical answers expected! answer for blank 1:
Answers: 3
You know the right answer?
A sample of gallium bromide, GaBr3, weighing 0.165 g was dissolved in water and treated with silver...
Questions


Chemistry, 01.08.2019 06:50



Physics, 01.08.2019 06:50


Mathematics, 01.08.2019 06:50

Mathematics, 01.08.2019 06:50




Chemistry, 01.08.2019 06:50



History, 01.08.2019 06:50


Biology, 01.08.2019 06:50



History, 01.08.2019 06:50

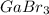 is 23.0%
is 23.0%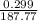 moles of AgBr =
moles of AgBr = 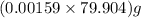 = 0.127 g
= 0.127 g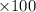 %
%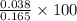 %
%

