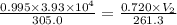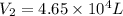Chemistry, 14.04.2020 17:50 jessica3981
A weather balloon is filled with helium that occupies a volume of 3.93 104 L at 0.995 atm and 32.0°C. After it is released, it rises to a location where the pressure is 0.720 atm and the temperature is -11.7°C. What is the volume of the balloon at that new location?

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 17:30
Observation and experimentation have led many scientists to accept a theory about the origin of the universe. this theory is called the big bang theory. scientific evidence collected and observed by scientists around the world suggests that the universe is ever expanding from a hot and dense initial state. what makes this a scientific theory? (2 points)
Answers: 2

Chemistry, 23.06.2019 09:30
What is the force of an object when it landed(sitting in the ground)
Answers: 2

Chemistry, 23.06.2019 10:00
1.9 mol hcl and 3.9 mol naoh react according to the equation hcl + naoh −→ nacl + h2o . if the limiting reactant is hcl, calculate the amount of nacl formed.
Answers: 1
You know the right answer?
A weather balloon is filled with helium that occupies a volume of 3.93 104 L at 0.995 atm and 32.0°C...
Questions



















Physics, 21.09.2019 00:10

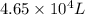
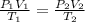
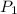 = initial pressure of gas = 0.995 atm
= initial pressure of gas = 0.995 atm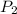 = final pressure of gas = 0.720 atm
= final pressure of gas = 0.720 atm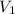 = initial volume of gas =
= initial volume of gas = 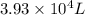
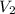 = final volume of gas = ?
= final volume of gas = ?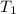 = initial temperature of gas =
= initial temperature of gas = 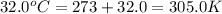
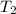 = final temperature of gas =
= final temperature of gas =