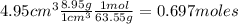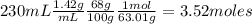Nitrogen dioxide is used industrially to produce nitric acid, but it contributes to acid rain and photochemical smog. What volume (in L) of nitrogen dioxide is formed at 735 torr and 28.2°C by reacting 4.95 cm3 of copper (d = 8.95 g/cm3 ) with 230.0 mL of nitric acid (d = 1.42 g/cm3 , 68.0% HNO3 by mass)?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:30
Based on its chemical properties, identify the position of each chemical family on the periodic table.
Answers: 3


Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning explain how a buffer works, using an ethanoic acid/sodium ethanoate system including how the system resists changes in ph upon addition of a small amount of base and upon addition of a small amount of acid respectively. include the following calculations in your i. calculate the ph of a solution made by mixing 25cm3 0.1m ch3cooh and 40cm3 0.1m ch3coo-na+. [ka = 1.74 x 10-5 m] ii. calculate the ph following the addition of a 10cm3 portion of 0.08 m naoh to 500cm3 of this buffer solution. iii. calculate the ph following the addition of a 10cm3 portion of 0.08 m hcl to 200cm3 of the original buffer solution.
Answers: 1

Chemistry, 22.06.2019 20:30
What is a difference between a mixture of elements and a mixture of compounds
Answers: 1
You know the right answer?
Nitrogen dioxide is used industrially to produce nitric acid, but it contributes to acid rain and ph...
Questions

Mathematics, 04.07.2019 11:10


English, 04.07.2019 11:10

Arts, 04.07.2019 11:10

History, 04.07.2019 11:10

Health, 04.07.2019 11:10


Health, 04.07.2019 11:10

Mathematics, 04.07.2019 11:10


Chemistry, 04.07.2019 11:10


Biology, 04.07.2019 11:10

Business, 04.07.2019 11:10

Mathematics, 04.07.2019 11:10


Mathematics, 04.07.2019 11:10

English, 04.07.2019 11:10

Mathematics, 04.07.2019 11:20

Mathematics, 04.07.2019 11:20