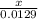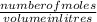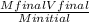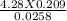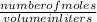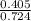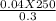Chemistry, 14.04.2020 07:44 aide1234564
1.Complete the balanced neutralization equation for the reaction below:
H2SO4 (aq) + Sr(OH)2 (aq) -->
2. How many L of a 0.209 M KI solution is needed to completely react with 2.43 g of Cu(NO3)2 according to the balanced chemical reaction:
2Cu(NO₃)₂(aq) + 4KI(aq) → 2CuI(aq) + I₂(s) + 4KNO₃(aq)
3.What volume in L of a 0.724 M Nal solution contains0.405 mol of Nal?
4. How many mL of 0.300 M NaF would be required to make a 0.0400 M solution of NaF when diluted to 250.0 mL with water?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 14:30
The three types is stress that act on earths rocks are compression, tension, and
Answers: 1

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 best answer will be brainliest
Answers: 3

Chemistry, 22.06.2019 18:30
Which rate indicates the number of children that would be born per woman if she were to live to the end of her child bearing years
Answers: 2

Chemistry, 23.06.2019 00:30
Maya wrote if you step to describe how carbon circulates between the atmosphere and living organisms
Answers: 1
You know the right answer?
1.Complete the balanced neutralization equation for the reaction below:
H2SO4 (aq) + Sr(OH)2 (...
H2SO4 (aq) + Sr(OH)2 (...
Questions



Mathematics, 09.10.2019 02:40

English, 09.10.2019 02:40


Mathematics, 09.10.2019 02:40




Mathematics, 09.10.2019 02:40

Biology, 09.10.2019 02:40


Mathematics, 09.10.2019 02:40

Mathematics, 09.10.2019 02:40

Physics, 09.10.2019 02:40


Mathematics, 09.10.2019 02:40



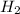 S
S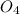 + Sr(OH)
+ Sr(OH) ⇒ SrSO4 + 2 H2O is the balanced reaction.
⇒ SrSO4 + 2 H2O is the balanced reaction.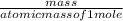
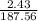
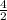 =
=