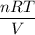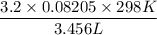Chemistry, 14.04.2020 06:12 faithlopez209
HEN
A 45.2 g sample of Nitrogen gas, N2, has a volume of 3,456mL and a
temperature of 25.0 °C. What is the pressure of the gas? *
F
O 1160 kPa
O 1.15 kPa
O 11.4 kPa
SES
EBRER
96.8 kPa

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Three cards with holes are arranged in a straight line. a light is shined through the first card’s hole and travels through all three cards. what does this tell you about light rays? a) that light is reflected b) that light is refractive c) that light travels in a straight line d) that light does not travel in a straight line
Answers: 1

Chemistry, 22.06.2019 15:30
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 1

Chemistry, 22.06.2019 18:00
Hydrogenation reactions, in which h2 and an "unsaturated" organic compound combine, are used in the food, fuel, and polymer industries. in the simplest case, ethene (c2h4) and h2 form ethane (c2h6). if 140 kj is given off per mole of c2h4 reacting, how much heat (in mj) is released when 12 kg of c2h6 forms?
Answers: 2

Chemistry, 23.06.2019 03:50
How many moles of potassium are needed to react completely with 12.8 moles of magnessium bromide?
Answers: 2
You know the right answer?
HEN
A 45.2 g sample of Nitrogen gas, N2, has a volume of 3,456mL and a
temperature of 25...
A 45.2 g sample of Nitrogen gas, N2, has a volume of 3,456mL and a
temperature of 25...
Questions


Mathematics, 30.11.2021 20:10


Business, 30.11.2021 20:10




Mathematics, 30.11.2021 20:10


English, 30.11.2021 20:10



SAT, 30.11.2021 20:10


Spanish, 30.11.2021 20:10

Mathematics, 30.11.2021 20:10

Mathematics, 30.11.2021 20:10

Mathematics, 30.11.2021 20:10

Mathematics, 30.11.2021 20:10

Social Studies, 30.11.2021 20:10

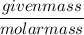 =
= 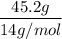 = 3.2 mol
= 3.2 mol