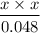Chemistry, 14.04.2020 04:27 lizdominguez101
Determine the pH of a 0.048 M hypochlorous acid (HClO) solution. Hypochlorous acid is a weak acid (Ka = 4.0 ✕ 10−8 M).

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1


Chemistry, 22.06.2019 10:30
Use this information to determine the number of calends electrons in the atoms. which of the following correctly compares the stability of the two atoms? a) both are unreactive b) both are highly reactive c) a is unreactive and d is reactive d) a is reactive and d is unreactive
Answers: 2

You know the right answer?
Determine the pH of a 0.048 M hypochlorous acid (HClO) solution. Hypochlorous acid is a weak acid (K...
Questions

Mathematics, 04.02.2021 21:00






History, 04.02.2021 21:00


Mathematics, 04.02.2021 21:00




World Languages, 04.02.2021 21:00

Arts, 04.02.2021 21:00




Mathematics, 04.02.2021 21:00

Mathematics, 04.02.2021 21:00

![$\frac{[H^{+}] [ClO^{-}] }{[HClO]}](/tpl/images/0597/7831/56a50.png)