6. which of the following statements is true?
A. in an exothermic reaction, the energy of the...

6. which of the following statements is true?
A. in an exothermic reaction, the energy of the products is the same as the energy of the reactants.
B. in an endothermic reaction, the energy of the products is the same as the energy of the reactants.
C. in an exothermic reaction, the energy of the products is less than the energy of the reactance.
D. in an endothermic reaction, the energy of the products is less than the energy of the reactants.
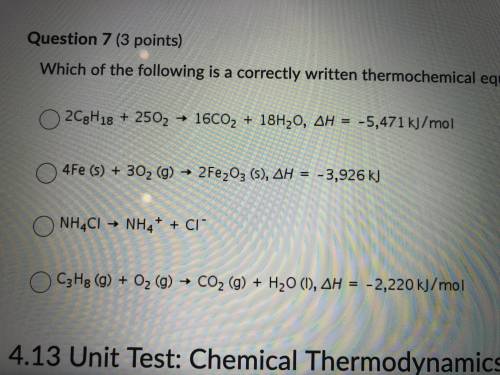
7. which of the following is correctly written thermochemical equation?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Match the following items. 1. high-intensity bundle of energy being emitted from some decaying nuclei gamma ray 2. particle radiating from the nucleus of some atoms beta particle 3. negative particle identical to an electron but radiating from a decaying nucleus alpha particle
Answers: 1


Chemistry, 22.06.2019 12:00
An atom's configuration based on its number of electrons ends at 3p4. another atom has seven more electrons. starting at 3p, what is the remaining configuration? 3p63d34s2 3p43d54s2 3p64s23d3 3p44s23d
Answers: 3

Chemistry, 22.06.2019 19:30
How might this scientific phenomena be explained? a paper clip floats on water.
Answers: 1
You know the right answer?
Questions



Mathematics, 20.08.2020 02:01


Mathematics, 20.08.2020 02:01

Computers and Technology, 20.08.2020 02:01

Mathematics, 20.08.2020 02:01


Mathematics, 20.08.2020 02:01





Arts, 20.08.2020 02:01



Biology, 20.08.2020 02:01






