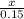Chemistry, 13.04.2020 18:33 gamerdoesart
A solution containing 20.0 g of sodium sulfite reacts with 7.0 ml of phosphoric acid. The concentration of the acid solution is such that there are 1.83 grams of H3PO4 per milliliter of solution. Determine the following: a. The mass of the excess reactant remaining at completion. b. Grams of water produced. c. Moles of sodium phosphate produced. d. Grams of sulfur dioxide produced.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:30
How many moles of carbon dioxide will form if 2.5 moles of c3h8 is burned
Answers: 1

Chemistry, 22.06.2019 14:00
Will mark brainliest how many electrons can be held in the energy level n = 4?
Answers: 1


Chemistry, 22.06.2019 20:00
I’m an electrically neutral atomic any element, there are equal numbers of
Answers: 2
You know the right answer?
A solution containing 20.0 g of sodium sulfite reacts with 7.0 ml of phosphoric acid. The concentrat...
Questions


Chemistry, 25.02.2021 03:30

Mathematics, 25.02.2021 03:30

Mathematics, 25.02.2021 03:30



Mathematics, 25.02.2021 03:30

Mathematics, 25.02.2021 03:30

Mathematics, 25.02.2021 03:30


English, 25.02.2021 03:30


Social Studies, 25.02.2021 03:30


Engineering, 25.02.2021 03:30

Mathematics, 25.02.2021 03:30




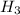 P
P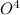 + 3
+ 3 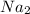
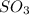 ⇒ 2
⇒ 2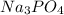 +3
+3 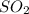 +3
+3 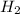 O
O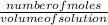
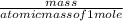
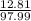
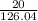
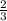 =
= 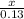
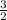 =
=