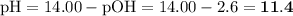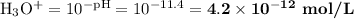Chemistry, 13.04.2020 01:10 alejandro8212003
Ammonia has a Kb of 1.8 × 10−5. Find [H3O+], [OH−], pH, and pOH for a 0.310 M ammonia solution.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Harvey kept a balloon with a volume of 348 milliliters at 25.0˚c inside a freezer for a night. when he took it out, its new volume was 322 milliliters, but its pressure was the same. if the final temperature of the balloon is the same as the freezer’s, what is the temperature of the freezer? the temperature of the freezer is kelvins.
Answers: 2

Chemistry, 22.06.2019 12:00
From the options provided for each element below, choose the properties that it may have based on its location in the periodic table fluorine (f): highly reactive nonmetal shiny a conductor
Answers: 1

Chemistry, 22.06.2019 15:00
Which theory was contradicted by experiments with the photoelectric effect? light spreads out after it passes through a small opening. as soon as light strikes metal, electrons will be ejected. visible light, regardless of color, will cause the ejection of electrons when striking metal. the kinetic energy of ejected electrons depends on the frequency of light that strikes the metal.
Answers: 2

Chemistry, 22.06.2019 20:00
Which of the following would not diffuse through the plasma membrane by means of simple diffusion? 1 oxygen 2 glucose 3 a steroid hormone 4 a lipid soluble vitamin
Answers: 3
You know the right answer?
Ammonia has a Kb of 1.8 × 10−5. Find [H3O+], [OH−], pH, and pOH for a 0.310 M ammonia solution....
Questions

History, 06.11.2019 07:31







Mathematics, 06.11.2019 07:31

Mathematics, 06.11.2019 07:31

Biology, 06.11.2019 07:31


Mathematics, 06.11.2019 07:31



Chemistry, 06.11.2019 07:31



History, 06.11.2019 07:31

History, 06.11.2019 07:31

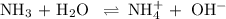

![K_{\text{b}} = \dfrac{\text{[BH}^{+}]\text{[OH}^{-}]}{\text{[B]}} = 1.8 \times 10^{-5}\\\\\dfrac{x^{2}}{0.100 - x} = 1.8 \times 10^{-5}](/tpl/images/0596/0459/4767e.png)
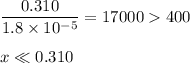
![\dfrac{x^{2}}{0.310} = 1.8 \times 10^{-5}\\\\x^{2} = 0.310 \times 1.8 \times 10^{-5}\\x^{2} = 5.58 \times 10^{-6}\\x = \sqrt{5.58 \times 10^{-6}}\\x = \text{[OH]}^{-} = \mathbf{2.4 \times 10^{-3}} \textbf{ mol/L}](/tpl/images/0596/0459/7dcd2.png)
![\text{pOH} = -\log \text{[OH}^{-}] = -\log(2.4 \times 10^{-3}) = \mathbf{2.6}](/tpl/images/0596/0459/b8dea.png)