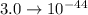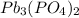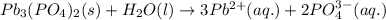The value of the Solubility Product Constant for lead phosphate is
Write the reaction t...

Chemistry, 11.04.2020 04:50 angelinaavila06
The value of the Solubility Product Constant for lead phosphate is
Write the reaction that corresponds to this Ksp value.
(Aq, S,L) +(Aq, S,L) <>(Aq, S,L) +(Aq, S,L)
Ksp values are found by clicking on the "Tables" link.
Use the pull-down menus to specify the state of each reactant or product.
If a box is not needed leave it blank.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 12:30
Which statement best describes how atoms combine to form sodium chloride (nacl)? a. a positively charged sodium ion and a positively charged chlorine ion form an covalent bond. b. a positively charged sodium ion and a negatively charged chlorine ion form an covalent bond. c. a positively charged sodium ion and a positively charged chlorine ion form an ionic bond. d. a positively charged sodium ion and a negatively charged chlorine ion form an ionic bond.
Answers: 1

Chemistry, 21.06.2019 16:50
If a substance is not at its melting or boiling point, as the heat content of a sample of matter increases, its temperature increases the number of intermolecular bonds decreases the space between particles increases the particles move faster
Answers: 2

Chemistry, 21.06.2019 23:00
Plz choose one of the compounds from the table and explain how you know the numbers of atoms in your formula. is it possible for two different compounds to be made from the exact same two elements? why or why not? with a limited number of elements (less than 120 are known), does this mean we also have a small number of compounds or do we have a large number of compounds in this world?
Answers: 1

Chemistry, 22.06.2019 08:30
How would the number of moles (n) of o2 change if the atmospheric pressure doubled but all other variables stayed the same
Answers: 2
You know the right answer?
Questions

Mathematics, 17.06.2021 20:30

Mathematics, 17.06.2021 20:30


History, 17.06.2021 20:30



Mathematics, 17.06.2021 20:30

Business, 17.06.2021 20:30


Mathematics, 17.06.2021 20:30

English, 17.06.2021 20:30

Mathematics, 17.06.2021 20:30




Engineering, 17.06.2021 20:30


Mathematics, 17.06.2021 20:30


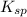 value of lead phosphate is given below and the value of solubility product for the same is
value of lead phosphate is given below and the value of solubility product for the same is