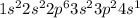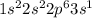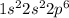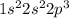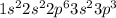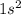Chemistry, 11.04.2020 04:03 browneyedbaby20
Which of the following electron configurations represents an excited state of the indicated atom? Group of answer choices
a. Na: 1s2 2s2 2p6 3s2 3p2 3s1
b. Ne: 1s2 2s2 2p6
c. N: 1s2 2s2 2p3
d. P: 1s2 2s2 2p6 3s2 3p2 4s1
e. He: 1s2

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:20
Which of the following is true for the actual yield of a reaction? it is always calculated as a ratio. it is the yield from the excess reactant. it is the yield from the limiting reactant. it is always less than the theoretical yield.
Answers: 1

Chemistry, 22.06.2019 12:30
According to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? view available hint(s) according to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? trigonal bipyramidal tetrahedral square planar determination of electron geometry requires information on whether the electron groups are lone pairs or bonding groups.
Answers: 2

Chemistry, 22.06.2019 12:30
The melting point of sulfur is 115 °c and its boiling point is 445 °c. what state would sulfur be in at 200 °c?
Answers: 1

You know the right answer?
Which of the following electron configurations represents an excited state of the indicated atom? Gr...
Questions

History, 16.10.2020 16:01

Biology, 16.10.2020 16:01

History, 16.10.2020 16:01





History, 16.10.2020 16:01


Social Studies, 16.10.2020 16:01

Mathematics, 16.10.2020 16:01

Biology, 16.10.2020 16:01

English, 16.10.2020 16:01




Mathematics, 16.10.2020 16:01

Chemistry, 16.10.2020 16:01


English, 16.10.2020 16:01