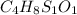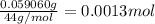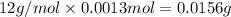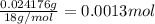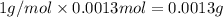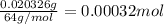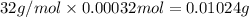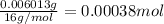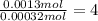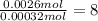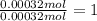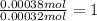A 33.153 mg sample of a chemical known to contain only carbon, hydrogen, sulfur, and oxygen is put into a combustion analysis apparatus, yielding 59.060 mg of carbon dioxide and 24.176 mg of water. In another experiment, 47.029 mg of the compound is reacted with excess oxygen to produce 20.326 mg of sulfur dioxide. Add subscripts below to correctly identify the empirical formula of this compound (use this order of elements: CHSO)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:00
Which of the following is not a good technique for managing used oil? a) have specific, labeled catch pans available for technicians who are collecting oil b) spills in your shop and any releases on pavement or outside should be poured down a drain c) do not use oil containers for antifreeze or other non-similar fluids d) be prepared for oil spills with the proper absorbents
Answers: 1

Chemistry, 22.06.2019 14:30
How does a noncompetitive inhibitor reduce an enzyme’s activity?
Answers: 1

Chemistry, 22.06.2019 21:00
Rays from the sun are not considered matter true or false
Answers: 2

Chemistry, 22.06.2019 23:40
The kw for water at 0 °c is 0.12× 10–14 m2. calculate the ph of a neutral aqueous solution at 0 °c.
Answers: 2
You know the right answer?
A 33.153 mg sample of a chemical known to contain only carbon, hydrogen, sulfur, and oxygen is put i...
Questions


Biology, 07.01.2021 17:40


Mathematics, 07.01.2021 17:40



Mathematics, 07.01.2021 17:40



English, 07.01.2021 17:40





Mathematics, 07.01.2021 17:40




Mathematics, 07.01.2021 17:40