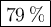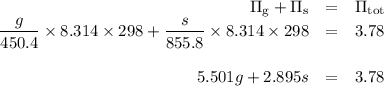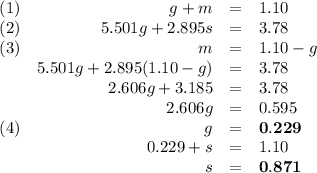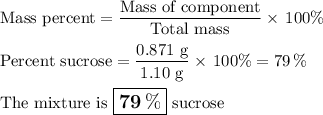Chemistry, 11.04.2020 00:53 alyssatamayo641
A 1.10 g sample contains only glucose and sucrose. When the sample is dissolved in water to a total solution volume of 25.0L, the osmotic pressure of the solution is 3.78 atm at 298k. What is mass percent sucrose?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:30
How many atoms of oxygen are contained in 160 grams of n2o3
Answers: 2

Chemistry, 21.06.2019 18:50
Problem page gaseous ethane reacts with gaseous oxygen gas to produce gaseous carbon dioxide and gaseous water . if of water is produced from the reaction of of ethane and of oxygen gas, calculate the percent yield of water. be sure your answer has the correct number of significant digits in it.
Answers: 2

Chemistry, 21.06.2019 19:10
Nuclear fusion is the source of energy for stars. besides hydrogen, which other element is most likely also common in stars?
Answers: 1

Chemistry, 22.06.2019 02:00
In which of these cases are the two wave points considered to be in phase with each other?
Answers: 1
You know the right answer?
A 1.10 g sample contains only glucose and sucrose. When the sample is dissolved in water to a total...
Questions

Mathematics, 05.01.2021 06:30


Mathematics, 05.01.2021 06:30


Mathematics, 05.01.2021 06:30

Spanish, 05.01.2021 06:30

English, 05.01.2021 06:30

English, 05.01.2021 06:30


English, 05.01.2021 06:30




Mathematics, 05.01.2021 06:30



English, 05.01.2021 06:30

History, 05.01.2021 06:30


Mathematics, 05.01.2021 06:30