

Answers: 2


Another question on Chemistry


Chemistry, 21.06.2019 22:50
Blank allows you to do calculations for situations in which only the amount of gas is constant a)boyle's law b)combined gas law c)ideal gas law d)dalton's law
Answers: 1

Chemistry, 22.06.2019 07:00
In the cathode ray tube experiment, j. j. thomson passed an electric current through different gases inside a cathode ray tube in the presence of an electric field. in which two ways did this experiment change scientists’ understanding of the atom?
Answers: 2

Chemistry, 22.06.2019 09:50
What are four significant sources of ghgs that come from wostem washington?
Answers: 2
You know the right answer?
Calculate the number of moles of oxygen gas that are required to react with 4.74 mol dodecane, C 10H...
Questions

Biology, 24.09.2019 23:30

History, 24.09.2019 23:30



Mathematics, 24.09.2019 23:30


Mathematics, 24.09.2019 23:30


History, 24.09.2019 23:30

History, 24.09.2019 23:30


Geography, 24.09.2019 23:30

Advanced Placement (AP), 24.09.2019 23:30

Mathematics, 24.09.2019 23:30


Physics, 24.09.2019 23:30


Social Studies, 24.09.2019 23:30

History, 24.09.2019 23:30

History, 24.09.2019 23:30

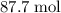 .
. 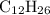 . (In an alkane molecule with
. (In an alkane molecule with 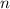 carbon atoms, there would be
carbon atoms, there would be 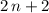 hydrogen atoms.)
hydrogen atoms.)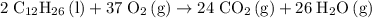 .
.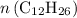 (the number of moles of dodecane in the reaction) and is asking for
(the number of moles of dodecane in the reaction) and is asking for 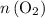 (the number of moles of oxygen in this reaction.) It would be helpful if there is a ratio
(the number of moles of oxygen in this reaction.) It would be helpful if there is a ratio 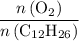 for directly converting
for directly converting 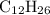 is
is 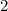 , andthe coefficient of
, andthe coefficient of 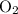 is
is 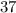 .
.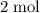 of
of 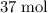 . Note that in this example, there's nothing special about the quantity of one mole. It is possible to scale the quantities of both reactants, and this ratio would still hold for this reaction. Thus,
. Note that in this example, there's nothing special about the quantity of one mole. It is possible to scale the quantities of both reactants, and this ratio would still hold for this reaction. Thus, 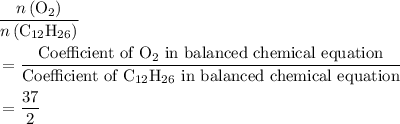 .
.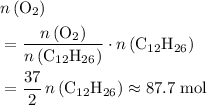 .
.

