
Chemistry, 11.04.2020 00:00 clevelandjaniya
Use this balanced equation to help you solve:
1. If 842 grams of sodium hydroxide reacts with 750.0 grams of aluminum, how many grams of aluminum hydroxide should theoretically form?
2. If only 512 grams actually form, what was the % yield? (Briefly describe the steps you would take to solve).
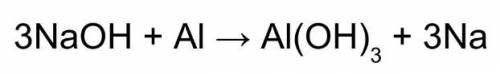

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 20:30
Identify the correct mole ratio for each substance. sodium chloride (nacl) na: cl = 1: ammonium nitrate (nhno) h: o = 4:
Answers: 1

Chemistry, 23.06.2019 02:50
Dumbledore decides to gives a surprise demonstration. he starts with a hydrate of na2co3 which has a mass of 4.31 g before heating. after he heats it he finds the mass of the anhydrous compound is found to be 3.22 g. he asks everyone in class to determine the integer x in the hydrate: na2co3·xh2o; you should do this also. round your answer to the nearest integ
Answers: 2

Chemistry, 23.06.2019 06:00
Which change will decrease the number of effective collisions during a chemical reaction? a. adding a catalyst b. increasing the surface area c. decreasing the temperature d. increasing the reactant concentrations e. increasing the volume of the reactants
Answers: 2

Chemistry, 23.06.2019 07:30
Which of the following statements best explains why chemistry is testable a) it can measure data by experiments b) it cannot add new evidence c) it cannot be verified d) it is biased
Answers: 1
You know the right answer?
Use this balanced equation to help you solve:
1. If 842 grams of sodium hydroxide reacts with...
1. If 842 grams of sodium hydroxide reacts with...
Questions


Mathematics, 26.03.2021 06:10



Business, 26.03.2021 06:10

World Languages, 26.03.2021 06:10

Mathematics, 26.03.2021 06:10

Mathematics, 26.03.2021 06:10



Mathematics, 26.03.2021 06:10




Business, 26.03.2021 06:10

English, 26.03.2021 06:10

Geography, 26.03.2021 06:10



Biology, 26.03.2021 06:10

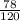 g of Al(OH)₃
g of Al(OH)₃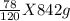 of Al(OH)₃
of Al(OH)₃


