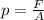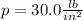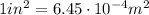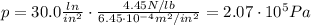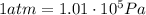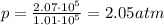The pressure in car tires is often measured in pounds per square inch (lb/in.2), with the recommended pressure being in the range of 25 to 45 lb/in.2. Suppose a tire has a pressure of 30.0 lb/in.2. Convert 30.0 lb/in.2 to its equivalent in atmospheres. Express the pressure numerically in atmospheres.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.036m naoh best answer will be brainliest
Answers: 3

Chemistry, 22.06.2019 22:30
The vapor pressure of ethanol is 1.00 × 102 mmhg at 34.90°c. what is its vapor pressure at 61.61°c? (δhvap for ethanol is 39.3 kj/mol.)
Answers: 2

Chemistry, 22.06.2019 23:00
How does the value of the equilibrium constant show that a reaction reaches equilibrium very quickly? (a) the equilibrium constant is large. (b) the equilibrium constant is small. (c) the equilibrium constant is zero. (d) the value of the equilibrium constant does not show how quickly a reaction comes to equilibrium.
Answers: 1

You know the right answer?
The pressure in car tires is often measured in pounds per square inch (lb/in.2), with the recommende...
Questions

English, 26.10.2020 19:40

History, 26.10.2020 19:40





Mathematics, 26.10.2020 19:40

Biology, 26.10.2020 19:40


Mathematics, 26.10.2020 19:40

Mathematics, 26.10.2020 19:40

Chemistry, 26.10.2020 19:40


Mathematics, 26.10.2020 19:40

Mathematics, 26.10.2020 19:40


Mathematics, 26.10.2020 19:40


Mathematics, 26.10.2020 19:40