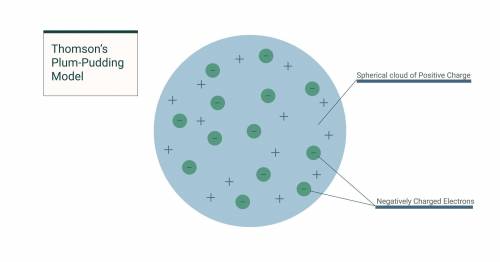
Which of the following answer choices best describes J. J. Thomson's plum-pudding model?An atom is surrounded by a firm outer shell and contains positively charged particles in its core. An atom is surrounded by a firm outer shell and contains negatively charged particles in its core. An atom consists of positively charged matter that contains negatively charged particles. An atom consists of negatively charged matter that contains positively charged particles.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:00
Asap! will give brainiest when a heat wave strikes a region causing more people to run air-conditioning units, electrical demand increases. what needs to be done to meet this increased demand? raising the control rodslowering the control rodsremoving the control rods
Answers: 1

Chemistry, 22.06.2019 16:30
At 20°c, a sample of h2o liquid and a sample of co2 gas each have the same average kinetic energy. why is one a liquid and the other a gas at this temperature?
Answers: 1

Chemistry, 22.06.2019 21:00
Read "who built the pyramids? ”. leave this link open while you answer the questions throughout the assignment. give at least two reasons why some people claim the pyramids of giza were constructed by aliens.
Answers: 1

Chemistry, 22.06.2019 22:30
Which is a characteristic of the electron sea model for metallic bonding? molecular orbitals overlap to produce bands. electrons flow easily between metal nuclei. electrons are in fixed positions in the orbitals. atomic nuclei are arranged in an irregular pattern.
Answers: 3
You know the right answer?
Which of the following answer choices best describes J. J. Thomson's plum-pudding model?An atom is s...
Questions



Mathematics, 15.01.2020 01:31

Geography, 15.01.2020 01:31


Mathematics, 15.01.2020 01:31

Computers and Technology, 15.01.2020 01:31

Chemistry, 15.01.2020 01:31


Physics, 15.01.2020 01:31

Mathematics, 15.01.2020 01:31


Biology, 15.01.2020 01:31

Mathematics, 15.01.2020 01:31




Mathematics, 15.01.2020 01:31


Mathematics, 15.01.2020 01:31