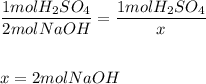41. To neutralize 1 mol of sulfuric acid, 2 mol of
sodium hydroxide are required. How many lit...


Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 15:50
In a popular classroom demonstration, solid sodium is added to liquid water and reacts to produce hydrogen gas and aqueous sodium hydroxide. part a write a balanced chemical equation for this reaction. express your answer as a chemical equation. identify all of the phases in your answer.
Answers: 3

Chemistry, 21.06.2019 22:30
Often on a topographic map, every fifth contour line is darkened. what is this line called? a. key b.slope c.benchmark d. index contour
Answers: 1

Chemistry, 21.06.2019 22:30
1.aluminum chloride (alcl3), and sodium hydroxide (naoh) can react to form aluminum hydroxide (al(oh)3) and sodium chloride (nacl). you have 13.4 g of aluminum chloride and 10.0 g of sodium hydroxide. answer the following questions: •what is the balanced equation for this reaction? •if you use all 13.4 g of aluminum chloride, how many grams of aluminum hydroxide can be formed? work must be shown to earn credit •if you use all 10.0 g of sodium hydroxide, how many grams of aluminum hydroxide can be formed? work must be shown to earn credit •how many grams of aluminum hydroxide will actually be made? which reagent is limiting? explain your answer.
Answers: 1

Chemistry, 22.06.2019 05:50
Calculate the number of molecules present in 0.750 mol of mgo.
Answers: 3
You know the right answer?
Questions


Mathematics, 18.08.2019 23:30

Mathematics, 18.08.2019 23:30

History, 18.08.2019 23:30


Mathematics, 18.08.2019 23:30

Social Studies, 18.08.2019 23:30


History, 18.08.2019 23:30


Mathematics, 18.08.2019 23:30


Biology, 18.08.2019 23:30

History, 18.08.2019 23:30

History, 18.08.2019 23:30



Mathematics, 18.08.2019 23:30

Mathematics, 18.08.2019 23:30

Mathematics, 18.08.2019 23:30