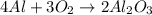Chemistry, 10.04.2020 04:56 katiedurden02
A 0.191g piece of solid aluminum reacts with gaseous oxygen from the atmosphere to form solid aluminum oxide. In the laboratory, a student weighs the mass of the aluminum oxide collected from this reaction as 0.199 g. The 0.191g solid aluminum is the .A) percent yield,
B) excess reagent,
C) limiting reagent,
D) actual yield,
E) theoretical yield

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:30
An empty fuel tank can still contain and therefore can be even more dangerous than one full of liquid fuel.
Answers: 1


Chemistry, 22.06.2019 12:40
When 13.3 g koh is dissolved in 102.7 g of water in a coffee-cup calorimeter, the temperature rises from 21.4 °c to 31.53 °c. what is the enthalpy change per gram of koh (j/g) dissolved in the water? * take the density of water as 1.00 g/ml. * assume that the solution has a specific heat capacity of 4.18 j/g*k. enter to 1 decimal place. do not forget the appropriate sign /(+). canvas may auto-delete the (+) sign
Answers: 2

Chemistry, 22.06.2019 21:30
What is another way to determine mass times acceleration?
Answers: 1
You know the right answer?
A 0.191g piece of solid aluminum reacts with gaseous oxygen from the atmosphere to form solid alumin...
Questions

Social Studies, 10.01.2020 18:31

Mathematics, 10.01.2020 18:31

Social Studies, 10.01.2020 18:31

History, 10.01.2020 18:31

Social Studies, 10.01.2020 18:31

Mathematics, 10.01.2020 18:31

Mathematics, 10.01.2020 18:31





History, 10.01.2020 18:31

Geography, 10.01.2020 18:31


Social Studies, 10.01.2020 18:31


History, 10.01.2020 18:31

Mathematics, 10.01.2020 18:31

Mathematics, 10.01.2020 18:31

English, 10.01.2020 18:31