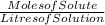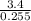Chemistry, 09.04.2020 22:27 clickbaitdxl
If I titrate an acid with 255mL of 3.4 M NaOH (base) and reach the equivalence point, how many moles of H+ ions were in the acid?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:00
How do scientists think that gravity affected the formation of our solar system?
Answers: 1

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 1

Chemistry, 22.06.2019 22:40
Percent ionization for a weak acid (ha) is determined by the following formula: percent ionization=[ha] ionized[ha] initial×100%for strong acids, ionization is nearly complete (100%) at most concentrations. however, for weak acids, the percent ionization changes significantly with concentration. the more diluted the acid is, the greater percent ionization.a certain weak acid, ha, has a ka value of 9.4×10? 7.part acalculate the percent ionization of ha in a 0.10 m solution.part bcalculate the percent ionization of ha in a 0.010 m solution
Answers: 1

You know the right answer?
If I titrate an acid with 255mL of 3.4 M NaOH (base) and reach the equivalence point, how many moles...
Questions

Mathematics, 23.12.2019 00:31

Business, 23.12.2019 00:31

Mathematics, 23.12.2019 00:31


History, 23.12.2019 00:31

Mathematics, 23.12.2019 00:31



Chemistry, 23.12.2019 00:31

History, 23.12.2019 00:31

Mathematics, 23.12.2019 00:31

Biology, 23.12.2019 00:31

Advanced Placement (AP), 23.12.2019 00:31



Chemistry, 23.12.2019 00:31

Mathematics, 23.12.2019 00:31