
Chemistry, 20.09.2019 06:20 jonmorton159
Which of the following statements concerning the reaction shown below are true? p4 (s) + 3o2 (g) → p4o6 (s) ∆h = -1640 kj i. heat is absorbed ii. heat is released iii. rxn is exothermic iv. rxn is endothermic v. products have higher enthalpy content than reactants vi. reactants have higher enthalpy content than products

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:30
The characteristic of two different types of reactions are shown below. reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of and element. which statement is true about the atoms of the elements that participate in the two reactions? a: their identity changes in both reaction a and b. b: their identity changes in reaction a but not b. c: their identity changes in reaction b but not a. d: their identity remains the same.
Answers: 1

Chemistry, 22.06.2019 17:30
I'm learning about the periodic tables and what each subject's configuration is. for example, hydrogen is 1s^1, but i don't understand how you get that. can someone me understand how to figure out how to figure this out? sorry if the question makes no sense, but it would really a lot if you could me understand! you so much if you can!
Answers: 1

Chemistry, 22.06.2019 18:30
Which rate indicates the number of children that would be born per woman if she were to live to the end of her child bearing years
Answers: 2

Chemistry, 22.06.2019 19:00
Sum of brother and sisters age is 26. four times the brothers age is subtracted from three times the sisters age, the difference is 8. what are the ages of the brother and sister?
Answers: 1
You know the right answer?
Which of the following statements concerning the reaction shown below are true? p4 (s) + 3o2 (g) →...
Questions







Biology, 05.05.2020 17:27

Chemistry, 05.05.2020 17:27





History, 05.05.2020 17:28



Mathematics, 05.05.2020 17:28


Social Studies, 05.05.2020 17:28

Social Studies, 05.05.2020 17:28

Mathematics, 05.05.2020 17:28

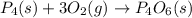
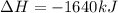
 for the reaction comes out to be negative.
for the reaction comes out to be negative.

