
Chemistry, 09.04.2020 19:25 sparky1234
A student puts 0.020 mol of methyl methanoate into an empty and rigid 1.0 L vessel at 450 K. The pressure is measured to be 0.74 atm. When the experiment is repeated using 0.020 mol of ethanoic acid instead of methyl methanoate, the measured pressure is lower than 0.74 atm. The lower pressure for ethanoic acid is due to the following reversible reaction. CH3COOH(g)+CH3COOH(g) ⇋ (CH3COOH)2(g)+Assume that when equilibrium has been reached, 50 percent of the ethanoic acid molecules have reacted. i. Calculate the total pressure in the vessel at equilibrium at 450 K. ii. Calculate the value of the equilibrium constant, Kp, for the reaction at 450 K

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:10
What shape would a molecule with two bound groups and two lone pairs have?
Answers: 1

Chemistry, 22.06.2019 20:10
Insoluble sulfide compounds are generally black in color. which of the following combinations could yield a black precipitate? check all that apply. na2s(aq)+kcl(aq) li2s(aq)+pb(no3)2(aq) pb(clo3)2(aq)+nano3(aq) agno3(aq)+kcl(aq) k2s(aq)+sn(no3)4(aq)
Answers: 1

Chemistry, 23.06.2019 00:30
When did stem cell research become known ? who discovered stem cell? what experiments or studies have been conducted so far?
Answers: 3

Chemistry, 23.06.2019 06:20
An object of mass 10.0 kg and volume 1000 ml and density 10 g/ml sinks in water who’s density is 1.0 g/ml. what is the mass of the water which has been displaced in kilograms
Answers: 1
You know the right answer?
A student puts 0.020 mol of methyl methanoate into an empty and rigid 1.0 L vessel at 450 K. The pre...
Questions



Social Studies, 20.10.2019 11:50


Health, 20.10.2019 11:50

History, 20.10.2019 11:50

History, 20.10.2019 11:50



Health, 20.10.2019 11:50


Mathematics, 20.10.2019 11:50


Arts, 20.10.2019 11:50


Mathematics, 20.10.2019 11:50

Biology, 20.10.2019 11:50



English, 20.10.2019 11:50

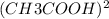 gas formed are calculated as
gas formed are calculated as


