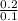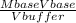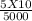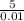Chemistry, 09.04.2020 03:57 ashley110608
A solution has [c6h5cooh] = 0.100 m and [ca(c6h5coo)2] = 0.200 m. ka = 6.3 × 10−5 for c6h5cooh. the solution volume is 5.00 l. what is the ph of the solution after 10.00 ml of 5.00 m naoh is added?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:00
1)each group 16 element has how many valence electrons? ( )4 ( )6 ( )8 ( )16 2)how many dots appear in the dot structure for calcium ion, ca2+? ( )zero ( )one ( )two ( )eight 3) which of the following atoms forms a cation to obtain an octet of outer shell electrons? ( )magnesium ( )oxygen ( )fluorine ( )helium 4) an al3+ ion contains 13 protons and 10 electrons. ( )true ( )false 5) valence and non-valence electrons are represented in lewis dot structures. ( )true ( )false
Answers: 3

Chemistry, 22.06.2019 12:40
Quiz1. which physical state of nitrogen has the highest entropy? a solid© b gasoc liquid
Answers: 1

Chemistry, 22.06.2019 20:00
The volume of a single vanadium atom is 9.29×10-24 cm3. what is the volume of a vanadium atom in microliters?
Answers: 3

Chemistry, 22.06.2019 21:30
Achemical reaction is done in the setup shown, resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 1
You know the right answer?
A solution has [c6h5cooh] = 0.100 m and [ca(c6h5coo)2] = 0.200 m. ka = 6.3 × 10−5 for c6h5cooh. the...
Questions


Biology, 05.07.2019 07:30


Mathematics, 05.07.2019 07:30

Mathematics, 05.07.2019 07:30

Biology, 05.07.2019 07:30




History, 05.07.2019 07:30

Biology, 05.07.2019 07:30

Social Studies, 05.07.2019 07:30

Mathematics, 05.07.2019 07:30


English, 05.07.2019 07:30

History, 05.07.2019 07:30

Mathematics, 05.07.2019 07:30



Mathematics, 05.07.2019 07:30

![\frac{[A-]}{[HA]}](/tpl/images/0591/3235/d82d4.png)