
Chemistry, 09.04.2020 03:31 jeisleen6808
2NH3(g) CO2(g) In an experiment carried out at this temperature, a certain amount of NH4OCONH2 is placed in an evacuated rigid container and allowed to come to equilibrium. Calculate the total pressure in the container at equilibrium.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Which statement describes evidence of a chemical reaction? a) ice melting eliminate b) water boiling c) lighting a match d) grape juice freezing
Answers: 3

Chemistry, 22.06.2019 04:30
Why are people not able to scuba dive in the deep part of the ocean
Answers: 2

Chemistry, 22.06.2019 06:30
Suppose a lab group reports a ppercent yield of sand of 105. is it really possible to collect more sand than was originally represented? what is the possible explanation for the extra product?
Answers: 2

Chemistry, 22.06.2019 10:10
Stage in which a star’s outer layers have started to cool and grow outward?
Answers: 3
You know the right answer?
2NH3(g) CO2(g) In an experiment carried out at this temperature, a certain amount of NH4OCONH2 is pl...
Questions

Biology, 29.08.2020 01:01

History, 29.08.2020 01:01


Mathematics, 29.08.2020 01:01




Mathematics, 29.08.2020 01:01


Social Studies, 29.08.2020 01:01


English, 29.08.2020 01:01







Mathematics, 29.08.2020 01:01

Mathematics, 29.08.2020 01:01

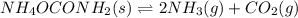
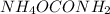 be 'x'
be 'x'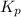 for above equation follows:
for above equation follows: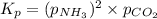
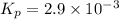
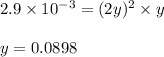
![p_{NH_3}+p_{CO_2}=[0.1796+0.0898]=0.2694atm](/tpl/images/0591/2646/0c04d.png)


