
Chemistry, 08.04.2020 22:29 ayoismeisalex
Given the balanced equation:
ZnSO4 + SrCl2 > SrSO4 + ZnCl2
What number of moles of SrCl2 is consumed when 54 g of ZnCl2 is produced?
a) 0.16 b) 0.3 c) 0.79 d) 1.58 e) 0.4

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:30
Order the following from smallest to largest atom, electron, quark, proton, neutron, molecule, nucleus
Answers: 1

Chemistry, 22.06.2019 12:20
Achemistry student weighs out 0.306 g of citric acid (h3c6h5o7), a triprotic acid, into a 250 ml volumetric flask and dilutes to the mark with distilled water. he plans to titrate the acid with 0.1000 m naoh solution. calculate the volume of naoh solution the student will need to add to reach the final equivalence point. be sure your answer has the correct number of significant digits.
Answers: 3

Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 1

You know the right answer?
Given the balanced equation:
ZnSO4 + SrCl2 > SrSO4 + ZnCl2
What number...
ZnSO4 + SrCl2 > SrSO4 + ZnCl2
What number...
Questions

Mathematics, 28.04.2021 01:30






Mathematics, 28.04.2021 01:30

Mathematics, 28.04.2021 01:30

Mathematics, 28.04.2021 01:30



Mathematics, 28.04.2021 01:30

Mathematics, 28.04.2021 01:30


Geography, 28.04.2021 01:30


English, 28.04.2021 01:30


Mathematics, 28.04.2021 01:30

History, 28.04.2021 01:30

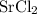 . consumed by 54 grams of zinc chloride has been 0.4 moles. Thus the correct option is e.
. consumed by 54 grams of zinc chloride has been 0.4 moles. Thus the correct option is e.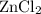 consumes 1 mole of
consumes 1 mole of 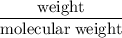
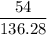 mol
mol

