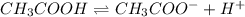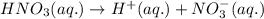Chemistry, 08.04.2020 21:58 rchapman414
Choose the statement below that is TRUE. Question 7 options: The term "strong electrolyte" means that the substance is extremely reactive. The term "weak electrolyte" means that the substance is inert. A weak acid solution consists of mostly nonionized acid molecules. A strong acid solution consists of only partially ionized acid molecules.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 21:30
Which of the following changes will decrease the total amount of gaseous solute able to be dissolved in a liter of liquid water? (2 points) decreasing temperature decreasing pressure decreasing surface area decreasing solute concentration
Answers: 1

Chemistry, 23.06.2019 08:50
Reacting masses1 calcium carbonate breaks down on heating to produce calcium oxide and carbondioxide gas.caco3 + cao + co2a student heats 15 g of calcium carbonate strongly in a crucible.relative atomic masses (a): ca = 40, c = 12, o = 16.calculate the mass of calcium oxide produced by this reaction.(5 marks)
Answers: 3

Chemistry, 23.06.2019 16:30
In chile, the deepest earthquake occurred at 61.7°w longitude at a depth of 540 km. if the rocks at the focus began subducting 10 million years ago and are now 1000 km from their original position, what is the average rate of subduction in cm/yr?
Answers: 1
You know the right answer?
Choose the statement below that is TRUE. Question 7 options: The term "strong electrolyte" means tha...
Questions

Mathematics, 18.11.2020 18:30

Biology, 18.11.2020 18:30



Physics, 18.11.2020 18:30


Biology, 18.11.2020 18:30


SAT, 18.11.2020 18:30




Mathematics, 18.11.2020 18:30


Physics, 18.11.2020 18:30


English, 18.11.2020 18:30

Business, 18.11.2020 18:30


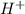 ions when dissolved in water. Thus most of molecules remain unionized in solutions.
ions when dissolved in water. Thus most of molecules remain unionized in solutions.