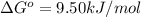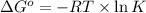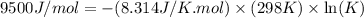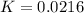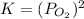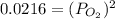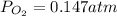Chemistry, 08.04.2020 21:39 audjwood67
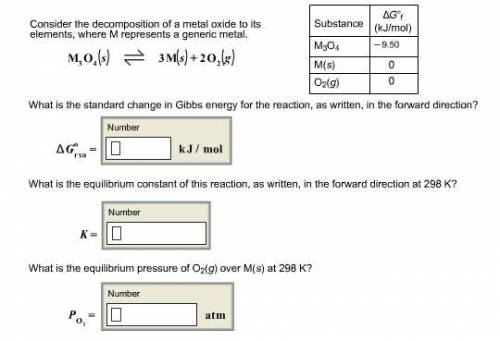
Consider the decomposition of a metal oxide to its elements, where M represents a generic metal. M 3 O 4 ( s ) − ⇀ ↽ − 3 M ( s ) + 2 O 2 ( g ) What is the standard change in Gibbs energy for the reaction, as written, in the forward direction? Δ G ∘ rxn = kJ/mol What is the equilibrium constant of this reaction, as written, in the forward direction at 298 K? K = What is the equilibrium pressure of O2(g) over M(s) at 298 K?

Answers: 2


Another question on Chemistry


Chemistry, 23.06.2019 01:00
Atoms contain subatomic particles called protons and neutrons. when these protons and neutrons spilt, a lot of energy is released
Answers: 3

Chemistry, 23.06.2019 04:10
An unknown substance has been shown to have weak covalent bonds. which of the following is most likely a property of this substance? a. high ph b. high conductivity c. low melting point d. low flammability
Answers: 3

Chemistry, 23.06.2019 05:30
Idont understand it 1.what is the boiling point of a solution of 675 grams of ethylene glycol (c2h6o2) in 2.50 liters of water?
Answers: 2
You know the right answer?
Consider the decomposition of a metal oxide to its elements, where M represents a generic metal. M 3...
Questions





Mathematics, 05.05.2020 08:37

History, 05.05.2020 08:37

Mathematics, 05.05.2020 08:37






Mathematics, 05.05.2020 08:37

Mathematics, 05.05.2020 08:37

Biology, 05.05.2020 08:37


English, 05.05.2020 08:37



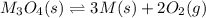
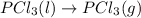
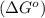 .
.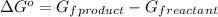
![\Delta G^o=[n_{M(s)}\times \Delta G^0_{(M(s))}+n_{O_2(g)}\times \Delta G^0_{(O_2(g))}]-[n_{M_3O_4(s)}\times \Delta G^0_{(M_3O_4(s))}]](/tpl/images/0590/3061/56a28.png)
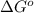 = Gibbs energy of reaction = ?
= Gibbs energy of reaction = ?![\Delta G^o=[3mole\times (0kJ/mol)+2mole\times (0kJ/mol)]-[1mole\times (-9.50kJ/K.mol)]](/tpl/images/0590/3061/a6722.png)