
Chemistry, 08.04.2020 21:35 charlesiarenee0
Consider the reaction H2(g) + I2(g) <-> HI(g) with an equilibrium constant of 46.3 and a reaction quotient of 525. Which direction will the system shift to?
The equilibrium will shift to the left to favor the reactants.
The equilibrium will shift to the right to favor the products.
The equilibrium will not shift in any direction.
The equilibrium will shift to the forward reaction.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 16:00
How will the volume of a gas be affected if the pressure is tripled, but the temperature remains the same?
Answers: 3

Chemistry, 22.06.2019 19:00
What information does a complete ionic equation give that the balanced equation doesn’t show?
Answers: 1

Chemistry, 22.06.2019 22:10
What is the indicator of the number of ions in solution? the amount of conductivity the amount of precipitate the amount of solute added
Answers: 1

Chemistry, 23.06.2019 00:40
To prevent the presence of air, noble gases are placed over highly reactive chemicals to act as inert "blanketing" gases. a chemical engineer places a mixture of noble gases consisting of 4.37 g of he, 13.36 g of ne, and 36.65 g of kr in a piston-cylinder assembly at stp. calculate the partial pressure in torr of kr.
Answers: 1
You know the right answer?
Consider the reaction H2(g) + I2(g) <-> HI(g) with an equilibrium constant of 46.3 and a react...
Questions


Mathematics, 08.12.2020 04:20


Mathematics, 08.12.2020 04:20



English, 08.12.2020 04:20

Law, 08.12.2020 04:20





Business, 08.12.2020 04:20



English, 08.12.2020 04:20




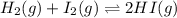
![Q=\frac{[HI]^2}{[H_2][I_2]}](/tpl/images/0590/2867/236ed.png)
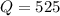

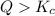 that means product > reactant. So, the reaction is reactant favored.
that means product > reactant. So, the reaction is reactant favored.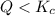 that means reactant > product. So, the reaction is product favored.
that means reactant > product. So, the reaction is product favored.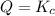 that means product = reactant. So, the reaction is in equilibrium.
that means product = reactant. So, the reaction is in equilibrium.

