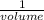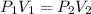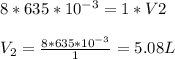Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:10
What approach is required to balance the objectives of sustainable development? balancing the objectives of sustainable development requires a(n) .
Answers: 3

Chemistry, 22.06.2019 03:40
Kc = 0.040 for the system below at 450oc. if a reaction is initiated with 0.40 mole of cl2 and 0.40 mole of pcl3 in a 2.0 liter container, what is the equilibrium concentration of cl2 in the same system? pcl5(g) ⇄ pcl3(g) + cl2(g)
Answers: 3

Chemistry, 22.06.2019 15:50
How many moles of potassium hydroxide are needed to completely react with 2.94 moles of aluminum sulfate
Answers: 1

Chemistry, 22.06.2019 16:30
For the reaction shown, calculate how many moles of no2 form when each of the following completely reacts. 2n2o5(g)→4no2(g)+o2(g) part a 1.0 mol n2o5 express your answer using two significant figures. nothing mol m o l request answer part b 5.4 mol n2o5 express your answer using two significant figures.
Answers: 2
You know the right answer?
635 mL of a gas is at a pressure of 8.00 atm. What is the volume of the gas at standard pressure (ST...
Questions

Mathematics, 19.11.2020 14:00

Mathematics, 19.11.2020 14:00



Mathematics, 19.11.2020 14:00


English, 19.11.2020 14:00

History, 19.11.2020 14:00


Computers and Technology, 19.11.2020 14:00

History, 19.11.2020 14:00

Mathematics, 19.11.2020 14:00

Mathematics, 19.11.2020 14:00

English, 19.11.2020 14:00



Social Studies, 19.11.2020 14:00

Geography, 19.11.2020 14:00