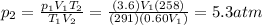Chemistry, 08.04.2020 19:24 pedroramirezr2
For many purposes we can treat nitrogen N2 as an ideal gas at temperatures above its boiling point of −196.°C. Suppose the temperature of a sample of nitrogen gas is lowered from 18.0°C to −15.0°C, and at the same time the pressure is changed. If the initial pressure was 3.6atm and the volume decreased by 40.0%, what is the final pressure? Round your answer to the correct number of significant digits.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Y=‐1x + 7 if y has a value of ‐24 what is the value of x?
Answers: 1


Chemistry, 22.06.2019 18:50
Question 3(multiple choice worth 4 points) (04.04 lc) what does it mean when an element is reduced? it empties a valance shell, reducing its atomic radius. it gains electrons, reducing its overall charge. it increases electronegativity, reducing its ability to bond. it loses electrons, reducing its electron number.
Answers: 1

Chemistry, 22.06.2019 21:30
How many oxygen atoms are there in 3.15 moles of hcl manganese (iv) oxide, mno2
Answers: 2
You know the right answer?
For many purposes we can treat nitrogen N2 as an ideal gas at temperatures above its boiling point o...
Questions

Business, 02.12.2020 20:00

Spanish, 02.12.2020 20:00

Mathematics, 02.12.2020 20:00




Biology, 02.12.2020 20:00

Mathematics, 02.12.2020 20:00

Mathematics, 02.12.2020 20:00


English, 02.12.2020 20:00

History, 02.12.2020 20:00


Mathematics, 02.12.2020 20:00


Mathematics, 02.12.2020 20:00

Biology, 02.12.2020 20:00



Mathematics, 02.12.2020 20:00

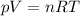

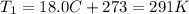 is the initial temperature of the gas
is the initial temperature of the gas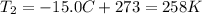 is the final temperature
is the final temperature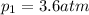 is the initial pressure
is the initial pressure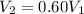 , as the volume is decreased by 40.0%
, as the volume is decreased by 40.0%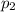 , we find the final pressure of the gas:
, we find the final pressure of the gas: