
Chemistry, 08.04.2020 19:04 vanydparis
Vinegar is a chemical used in cooking, cleaning and other common experiences. A 0.1 M solution of vinegar in water has a [H+] of about 1.3 × 10–3. (You may prefer to think of the hydronium ion concentration, [H3O+], as 1.3 × 10–3.)
A. Write the formula for the calculation of pH, and then show each step as you calculate the pH of a 0.1 M solution of vinegar.
B. Is vinegar an acid or a base? Explain how you know.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 23:30
Substance a is a nonpolar liquid and has only dispersion forces among its constituent particles. substance b is also a nonpolar liquid and has about the same magnitude of dispersion forces among its constituent particles. when substance a and b are combined, they spontaneously mix.
Answers: 1

Chemistry, 23.06.2019 00:30
What is bromine+calcium iodide--> calcium bromide +iodine balanced
Answers: 1

Chemistry, 23.06.2019 04:31
Pls i will do pls imma diewhat forms white light? (4 points)a. combination of all wavelengths of ultraviolet light b. combination of all wavelengths of visible lightc. absorption of electromagnetic waves d. absorption of infrared rays
Answers: 2

You know the right answer?
Vinegar is a chemical used in cooking, cleaning and other common experiences. A 0.1 M solution of vi...
Questions


Mathematics, 05.05.2020 11:39

Mathematics, 05.05.2020 11:39


Mathematics, 05.05.2020 11:39

Mathematics, 05.05.2020 11:39







Mathematics, 05.05.2020 11:39







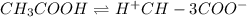
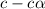
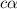
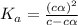
![[H^+]=c\times \alpha](/tpl/images/0589/7489/4fc41.png)
![[H^+]=0.1\times \alpha](/tpl/images/0589/7489/b5870.png)
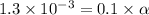
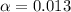
![pH=-log[H^+]](/tpl/images/0589/7489/15713.png)
![pH=-log[1.3\times 10^{-3}]=2.9](/tpl/images/0589/7489/92802.png)


