Chemistry, 08.04.2020 18:27 averyeverdeen01
What is the molar solubility of mn(oh)2(s) in a solution that is buffered at ph 8.00 at 25 °c? the ksp of mn(oh)2 is 1.9 ´ 10–13 at 25 °c?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:20
Calculate the molarity of 48.0 ml of 6.00 m h2so4 diluted to 0.250 l .
Answers: 1

Chemistry, 22.06.2019 20:10
Suppose you mix one mole of sulfuric acid (h2so4) with 1 mole of sodium hydroxide(naoh). why does the ph of the solution remain below 7? ( explain so i can get better understanding! )
Answers: 2

Chemistry, 23.06.2019 01:00
Na chemical reaction, activation energy increases the of the reactants. this outcome causes the particles to collide, which results in the of new products.
Answers: 2

Chemistry, 23.06.2019 07:30
Type the letter that represents the correct location for each particle type below. the neutron is found at __ the electron is found at __ the proton is found at __
Answers: 2
You know the right answer?
What is the molar solubility of mn(oh)2(s) in a solution that is buffered at ph 8.00 at 25 °c? the k...
Questions

English, 17.10.2021 15:00

Mathematics, 17.10.2021 15:00

English, 17.10.2021 15:00



Physics, 17.10.2021 15:00


Mathematics, 17.10.2021 15:10


Law, 17.10.2021 15:10

Social Studies, 17.10.2021 15:10

Geography, 17.10.2021 15:10

Law, 17.10.2021 15:10


Physics, 17.10.2021 15:10

English, 17.10.2021 15:10


Mathematics, 17.10.2021 15:10


English, 17.10.2021 15:10

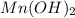 in a solution that is buffered at ph 8.00 is 0.19 M
in a solution that is buffered at ph 8.00 is 0.19 M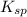
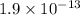
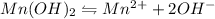
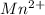 and 2 moles of
and 2 moles of 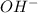
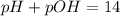
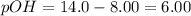
![6.00=-log[OH^-]](/tpl/images/0589/5690/6f655.png)
![[OH^-]=10^{-6}M](/tpl/images/0589/5690/2d0a2.png)
![K_{sp}=[Mn^{2+}][OH^{-}]^2](/tpl/images/0589/5690/25e3a.png)
![1.9\times 10^{-13}M=[s][(10^{-6})^2]](/tpl/images/0589/5690/ff545.png)