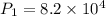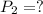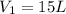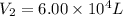Chemistry, 08.04.2020 17:17 clydeben4543
A 15.0 L tank of gas is contained at a high pressure of 8.20*10^4. The tank is opened and the gas expands into an empty chamber with a volume of 6.00*10^4 L. Calculate the new pressure of the gas.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 14:30
In water, a strong acid will break down into its component parts. a. completely b. partly c. never in water, a weak base will break down into its component parts. a. completely b. partly c. never
Answers: 2

Chemistry, 22.06.2019 22:00
The volume of an unknown substance in a sealed glass jar is 50 milliliters. the volume of the jar is 200 milliliters. which state of matter could the substance be?
Answers: 2

Chemistry, 23.06.2019 00:30
What is the percent by mass of magnesium sulfate in mgso4.7h2o
Answers: 3

Chemistry, 23.06.2019 03:20
High-pressure liquid chromatography (hplc) is a method used in chemistry and biochemistry to purify chemical substances. the pressures used in this procedure range from around 500 kilopascals (500,000 pa) to about 60,000 kpa (60,000,000 pa). it is often convenient to know the pressure in torr. if an hplc procedure is running at a pressure of 1.03×108 pa , what is its running pressure in torr?
Answers: 3
You know the right answer?
A 15.0 L tank of gas is contained at a high pressure of 8.20*10^4. The tank is opened and the gas ex...
Questions


Mathematics, 20.10.2020 18:01


Mathematics, 20.10.2020 18:01

English, 20.10.2020 18:01

History, 20.10.2020 18:01


Mathematics, 20.10.2020 18:01


English, 20.10.2020 18:01



Mathematics, 20.10.2020 18:01


History, 20.10.2020 18:01




World Languages, 20.10.2020 18:01

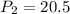
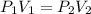 ..........1
..........1