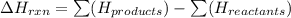Chemistry, 08.04.2020 04:43 pleasehelpme666
Consider the reaction: C2H5OH(ℓ) + 3O2(g) → 2CO2(g) + 3H2O(ℓ); ∆H = –1.37 x 103 kJ Consider the following statements: I. The reaction is endothermic II. The reaction is exothermic. III. The enthalpy term would be different if the water formed was gaseous. Which of these statement(s) is (are) true?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Ionic compounds are made of ions, and yet the overall charge of an ionic compound is neutral. why?
Answers: 1

Chemistry, 22.06.2019 07:20
Why does his teacher ask him to balance the equation by including the correct coefficient
Answers: 1

Chemistry, 22.06.2019 08:30
Which of the following would be an accurate picture of the earth during the summer time of the northern hemisphere?
Answers: 1

Chemistry, 22.06.2019 10:50
An atom of lithium-7 has an equal number of(1) electrons and neutrons(2) electrons and protons(3) positrons and neutrons(4) positrons and protons
Answers: 2
You know the right answer?
Consider the reaction: C2H5OH(ℓ) + 3O2(g) → 2CO2(g) + 3H2O(ℓ); ∆H = –1.37 x 103 kJ Consider the foll...
Questions



Mathematics, 18.12.2020 20:50

Mathematics, 18.12.2020 20:50

Chemistry, 18.12.2020 20:50

Chemistry, 18.12.2020 20:50

Chemistry, 18.12.2020 20:50



Mathematics, 18.12.2020 20:50




English, 18.12.2020 20:50

Chemistry, 18.12.2020 20:50

History, 18.12.2020 20:50

Mathematics, 18.12.2020 20:50

English, 18.12.2020 20:50

Mathematics, 18.12.2020 20:50