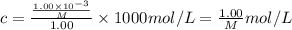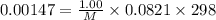A chemist is trying to determine the molar mass of a certain protein. 1.00 x 10 -3 g of it was dissolved in enough water to make 1.00 mL of solution. The osmotic pressure of this solution was found to be 1.12 torr at 25.0°C. Calculate the molar mass of the protein.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Asolution of sodium hydroxide was titrated against a solution of sulfuric acid. how many moles of sodium hydroxide would react with 1 mole of sulfuric acid?
Answers: 2


Chemistry, 22.06.2019 14:30
Consider the reduction reactions and their equilibrium constants. cu+(aq)+e−↽−−⇀cu(s)pb2+(aq)+2e−↽−−⇀pb(s)fe3+(aq)+3e−↽−−⇀fe(=6.2×108=4.0×10−5=9.3×10−3 cu + ( aq ) + e − ↽ − − ⇀ cu ( s ) k =6.2× 10 8 pb 2 + ( aq ) +2 e − ↽ − − ⇀ pb ( s ) k =4.0× 10 − 5 fe 3 + ( aq ) +3 e − ↽ − − ⇀ fe ( s ) k =9.3× 10 − 3 arrange these ions from strongest to weakest oxidizing agent.
Answers: 3

Chemistry, 22.06.2019 19:50
What is the wavelength of a wave with a velocity of 50 m/s and a frequency of 5hz a 250 m b 0.1 m c 10m d 0.01 m
Answers: 2
You know the right answer?
A chemist is trying to determine the molar mass of a certain protein. 1.00 x 10 -3 g of it was disso...
Questions

Advanced Placement (AP), 24.09.2020 02:01


Biology, 24.09.2020 02:01

Mathematics, 24.09.2020 02:01



History, 24.09.2020 02:01

Mathematics, 24.09.2020 02:01





History, 24.09.2020 02:01

Spanish, 24.09.2020 02:01




Health, 24.09.2020 02:01

History, 24.09.2020 02:01

Mathematics, 24.09.2020 02:01

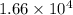 g/mol
g/mol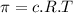
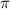 represents osmotic pressure of solution, c represents molarity of solution and T represents temperature in kelvin scale.
represents osmotic pressure of solution, c represents molarity of solution and T represents temperature in kelvin scale.