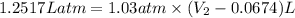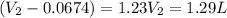Chemistry, 08.04.2020 04:34 xocupcake309174
A sample of gas occupies a volume of 67.4 mL 67.4 mL . As it expands, it does 126.8 J 126.8 J of work on its surroundings at a constant pressure of 783 Torr 783 Torr . What is the final volume of the gas?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:00
How many moles of magnesium is 3.01 x10^22 atoms of magnesium?
Answers: 1

Chemistry, 22.06.2019 04:30
The big bang nucleosynthesis theory states that elements were produced in the first few minutes of the big bang while elements have their origins in the interiors of stars, forming much later in the history of the universe.
Answers: 1

Chemistry, 22.06.2019 09:40
Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid synthesis. an industrial chemist studying this reaction fills a 25.0l tank with 4.5 mol of sulfur dioxide gas and 4.5 mol of oxygen gas at 30.°c. he then raises the temperature, and when the mixture has come to equilibrium measures the amount of sulfur trioxide gas to be 1.4 mol. calculate the concentration equilibrium constant for the reaction of sulfur dioxide and oxygen at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 3

Chemistry, 22.06.2019 11:00
The number to the right of an element's symbol (ex. c-12) identifies the of an isotope.
Answers: 1
You know the right answer?
A sample of gas occupies a volume of 67.4 mL 67.4 mL . As it expands, it does 126.8 J 126.8 J of wor...
Questions


Mathematics, 31.03.2021 01:30


Computers and Technology, 31.03.2021 01:30


Social Studies, 31.03.2021 01:30

Mathematics, 31.03.2021 01:30



Mathematics, 31.03.2021 01:30

English, 31.03.2021 01:30

Computers and Technology, 31.03.2021 01:30

Mathematics, 31.03.2021 01:30


Mathematics, 31.03.2021 01:30


History, 31.03.2021 01:30



Computers and Technology, 31.03.2021 01:30


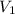 = initial volume = 67.4 ml = 0.0674L (1L=1000ml)
= initial volume = 67.4 ml = 0.0674L (1L=1000ml)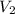 = final volume = ?
= final volume = ?