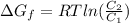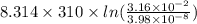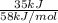Gastric juice (pH 1.5) is produced by pumping HCl from blood plasma (pH 7.4) into the stomach. Calculate the amount of free energy required to concentrate the H in 1 liter of gastric juice at 37 degree of centigrade. Under cellular conditions, how many moles of ATP must be hydrolyzed to provide this amount of free energy

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:00
Joe shines white light into a bowl half full of water at an angle of incident of 27.5°. calculate the angle of refraction in the water given the indices of refraction for air and water are 1.00 and 1.36, respectively.
Answers: 2

Chemistry, 22.06.2019 15:00
Many ionic compounds and a few highly polar covalent compounds are because they completely ionize in water to create a solution filled with charged ions that can conduct an electric current.
Answers: 1

Chemistry, 23.06.2019 00:30
If there are 3.5 moles of koh, how many moles of naoh can be produced? question 1 options: a)3.0 moles naoh b)3.5 moles naoh c)1 moles naoh d)9 moles naoh
Answers: 1

Chemistry, 23.06.2019 04:20
The lewis diagrams for magnesium and fluorine are shown below. what is the correct chemical formula for magnesium fluoride? a. mgf b. mg2f c. mgf2 d. mg2f3
Answers: 1
You know the right answer?
Gastric juice (pH 1.5) is produced by pumping HCl from blood plasma (pH 7.4) into the stomach. Calcu...
Questions



Geography, 02.04.2021 08:50

Social Studies, 02.04.2021 08:50

Mathematics, 02.04.2021 08:50


Mathematics, 02.04.2021 08:50


Biology, 02.04.2021 08:50

Mathematics, 02.04.2021 08:50

Mathematics, 02.04.2021 08:50

Mathematics, 02.04.2021 08:50

Mathematics, 02.04.2021 08:50




Computers and Technology, 02.04.2021 09:00

Mathematics, 02.04.2021 09:00

Mathematics, 02.04.2021 09:00

Mathematics, 02.04.2021 09:00

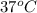 = (37 + 273) K
= (37 + 273) K![-log [H^{+}]](/tpl/images/0588/8520/822be.png)
![[H^{+}]](/tpl/images/0588/8520/85507.png) as follows.
as follows.![[H^{+}] = 10^{-pH}](/tpl/images/0588/8520/241df.png)
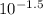
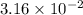 M (
M (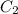 )
)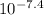
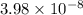 M (
M (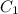 )
)