
Chemistry, 08.04.2020 03:32 LordBooming
For which of the following reactions is S° > 0. Choose all that apply. NH4I(s) NH3(g) + HI(g) CH4(g) + H2O(g) CO(g) + 3H2(g) 2NH3(g) + 3N2O(g) 4N2(g) + 3H2O(g) 2NH3(g) + 2O2(g) N2O(g) + 3H2O(l) CO(g) + Cl2(g) COCl2(g)

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:20
One or more substances changing into one or more substances is an example of a
Answers: 1

Chemistry, 22.06.2019 02:10
Determine the percent sulfuric acid by mass of a 1.61 m aqueous solution of h2so4. %
Answers: 2

Chemistry, 22.06.2019 02:20
Compared with the freezing-point depression of a 0.01 m c6h12o6 solution, the freezing-point depression of a 0.01 m nacl solution is
Answers: 1

Chemistry, 22.06.2019 07:00
At 450 mm hg a gas has a volume of 760 l, what is its volume at standard pressure
Answers: 2
You know the right answer?
For which of the following reactions is S° > 0. Choose all that apply. NH4I(s) NH3(g) + HI(g) CH4...
Questions


Mathematics, 02.06.2021 02:20





History, 02.06.2021 02:20


Advanced Placement (AP), 02.06.2021 02:20


Spanish, 02.06.2021 02:20



Health, 02.06.2021 02:20



History, 02.06.2021 02:20

Mathematics, 02.06.2021 02:20


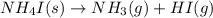
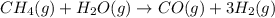
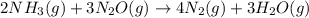
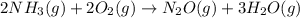
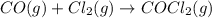
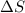 > 0.
> 0.

