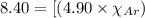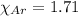Chemistry, 08.04.2020 03:11 makenahbriana
In a gas mixture of He, Ne, and Ar with a total pressure of 8.40 atm, the mole fraction of Ar is if the partial pressures of He and Ne are 1.50 and 2.00 atm, respectively.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 14:00
Displacement is the slope of a velocity vs. time graph a. true b. false
Answers: 1


Chemistry, 22.06.2019 23:30
If it is an isoelectronic series select true, if not select false. o2-, s2-, se2-, te2- na+, k+, rb+, cs+ n3-, p3-, as3-, sb3- ag, cd+, sn3+, sb4+ f-, cl-, br-, i- f-, ne, na+, mg2+ s2-, s, s6+
Answers: 1
You know the right answer?
In a gas mixture of He, Ne, and Ar with a total pressure of 8.40 atm, the mole fraction of Ar is if...
Questions

Chemistry, 14.10.2019 17:10

English, 14.10.2019 17:10


Mathematics, 14.10.2019 17:10

Mathematics, 14.10.2019 17:10


English, 14.10.2019 17:10


Biology, 14.10.2019 17:10

Health, 14.10.2019 17:10




Biology, 14.10.2019 17:10

Mathematics, 14.10.2019 17:10

English, 14.10.2019 17:10




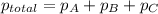
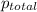 = total pressure of gases = 8.40 atm
= total pressure of gases = 8.40 atm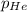 = partial pressure of helium = 1.50 atm
= partial pressure of helium = 1.50 atm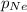 = partial pressure of neon = 2.00 atm
= partial pressure of neon = 2.00 atm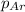 = partial pressure of argon = ?
= partial pressure of argon = ?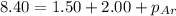
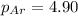
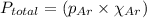
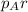 = partial pressure of argon = 4.90
= partial pressure of argon = 4.90 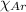 = mole fraction of argon = ?
= mole fraction of argon = ?