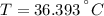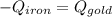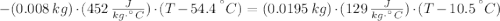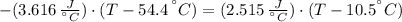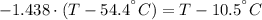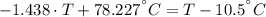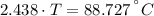Chemistry, 08.04.2020 02:07 tleppek6245
A sheet of gold weighing 8.8 g and at a temperature of 10.5°C is placed flat on a sheet of iron weighing 19.5 g and at a temperature of 54.4°C. What is the final temperature of the combined metals? Assume that no heat is lost to the surroundings.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Arollercoaster car at the top of a hill has potential energy kinetic energy chemical energy light energy
Answers: 1


Chemistry, 22.06.2019 11:50
The chemical bond connecting one nucleotide with the next one along the nucleic acid chain is called a
Answers: 3

Chemistry, 22.06.2019 12:00
Ican determine the molar mass of an element by looking on the under the atomic mass for the element. for example the molar mass of phosphorus is 30.974 grams/mole. avogadro’s number tells me the amount of representative particles in 1 mole of any substance. this means 12.011 gram sample of carbon and a 32.0 gram sample of sulfur have the same number of atoms.
Answers: 1
You know the right answer?
A sheet of gold weighing 8.8 g and at a temperature of 10.5°C is placed flat on a sheet of iron weig...
Questions


Advanced Placement (AP), 04.07.2019 14:10

Mathematics, 04.07.2019 14:10


Spanish, 04.07.2019 14:10

English, 04.07.2019 14:10




Spanish, 04.07.2019 14:10



History, 04.07.2019 14:10







Computers and Technology, 04.07.2019 14:10