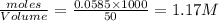Chemistry, 08.04.2020 01:53 Abdirisack3250
Calculate the mass of AgCl formed, and the concentration of silver ion remaining in solution, when 10.0g of solid AgNO3 is added to 50.mL of 1.0x10-2 Assume there is no volume change upon addition of the solid.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:10
Which of the following elements would you expect to have the lowest ionization energy value? fluorine, lithium, neon, nitrogen
Answers: 2

Chemistry, 22.06.2019 05:30
Astudent carefully transfers 30 g of water and 30 g of alcohol in a glass tube, forming two layers and filling the tube completely. after sealing the tube, the student mixes the solutions, and notices a bubble that forms in the tube. what is the mass of the contents in the glass tube after mixing?
Answers: 2

Chemistry, 22.06.2019 10:00
Part 1: include important facts found through your research. part 2: include your visual display. include your summary of “the chemistry of water” from the national science foundation website. include your experiment. part 3: include responses to the reflection questions.
Answers: 1

Chemistry, 22.06.2019 21:30
How many liters of 3.0 m naoh solution will react with 0.60 liters of 4.0 m h2so4? h2so4 + naoh → na2so4 + h2o 1.2 l 1.6 l 2.4 l 2.8 l
Answers: 3
You know the right answer?
Calculate the mass of AgCl formed, and the concentration of silver ion remaining in solution, when 1...
Questions

English, 29.11.2020 14:00

History, 29.11.2020 14:00

Mathematics, 29.11.2020 14:00

Mathematics, 29.11.2020 14:00


Mathematics, 29.11.2020 14:00

Mathematics, 29.11.2020 14:00

Mathematics, 29.11.2020 14:00

Mathematics, 29.11.2020 14:00

English, 29.11.2020 14:00

English, 29.11.2020 14:00

Mathematics, 29.11.2020 14:00

English, 29.11.2020 14:00





Mathematics, 29.11.2020 14:00


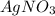 is added to 50.mL of
is added to 50.mL of 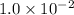 NaCl. Assume there is no volume change upon addition of the solid.
NaCl. Assume there is no volume change upon addition of the solid.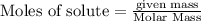
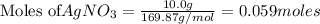
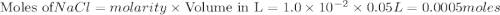
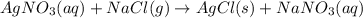
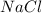 require = 1 mole of
require = 1 mole of 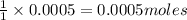 of
of 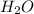
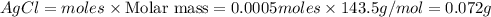
![[Ag]^+](/tpl/images/0588/5138/ed5fe.png) left in solution =
left in solution =